Right Sampling techniques for your Microbiological Analysis Environmental and Product Sampling
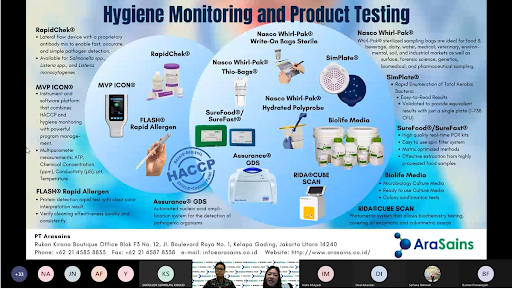
The webinar on Right Sampling techniques for Microbiological Analysis (Environmental and Product Sampling) was conducted jointly with Nasco Sampling/Whirl Pak Mr Jim, General Manager and Mr Andy Qin, Regional Sales Manager. This webinar was important because right sampling techniques were needed for good testing results. When the sampling fail, the testing would be compromised. Mr Jim talked in-depth about the variety of sampling tools available for QA/QC personnel to use which were sampling bags for products and sponges for swabbing pathogens from environment. He demonstrated the robustness of the WhirlPak bags, assuring customers that the bags are intact while in transport and storage.