Mycotoxin Detection
Mycotoxin
Mycotoxins are toxic chemical compounds produced by molds. There are several mycotoxins that has become a concern in industry; Aflatoxin B1, B2, G1, G2; Aflatoxin M1; Fumonisisn B1, B2, B3; Zearalenone; Ochratoxin A; etc. These mycotoxins can affect humans and animals because they are carcinogenic. PT. Arasains offer several kits that can help to detect mycotoxins using lateral flow (RIDA®QUICK), ELISA method (RIDASCREEN®) and immunoaffinity column (IAC) for HPLC.
International standards and code of practice for mycotoxins are established by codex allimentarius (www.fao.org/fileadmin/user_upload/agns/pdf/CXS_193e.pdf).
In the EU, the standards for mycotoxins are listed in the commission regulation EC No. 1881/2006 (eur-lex.europa.eu/legal-content/EN/TXT/).
In Indonesia, the standards for mycotoxins in food are listed in the BPOM regulation No. 8 Tahun 2018 (standarpangan.pom.go.id/dokumen/peraturan/2018/Salinan_PerBPOM_8_Tahun_2018_tentang_Cemaran_Kimia_Pangan_Join.pdf) and for feed are listed in the SNI 4483:2013.
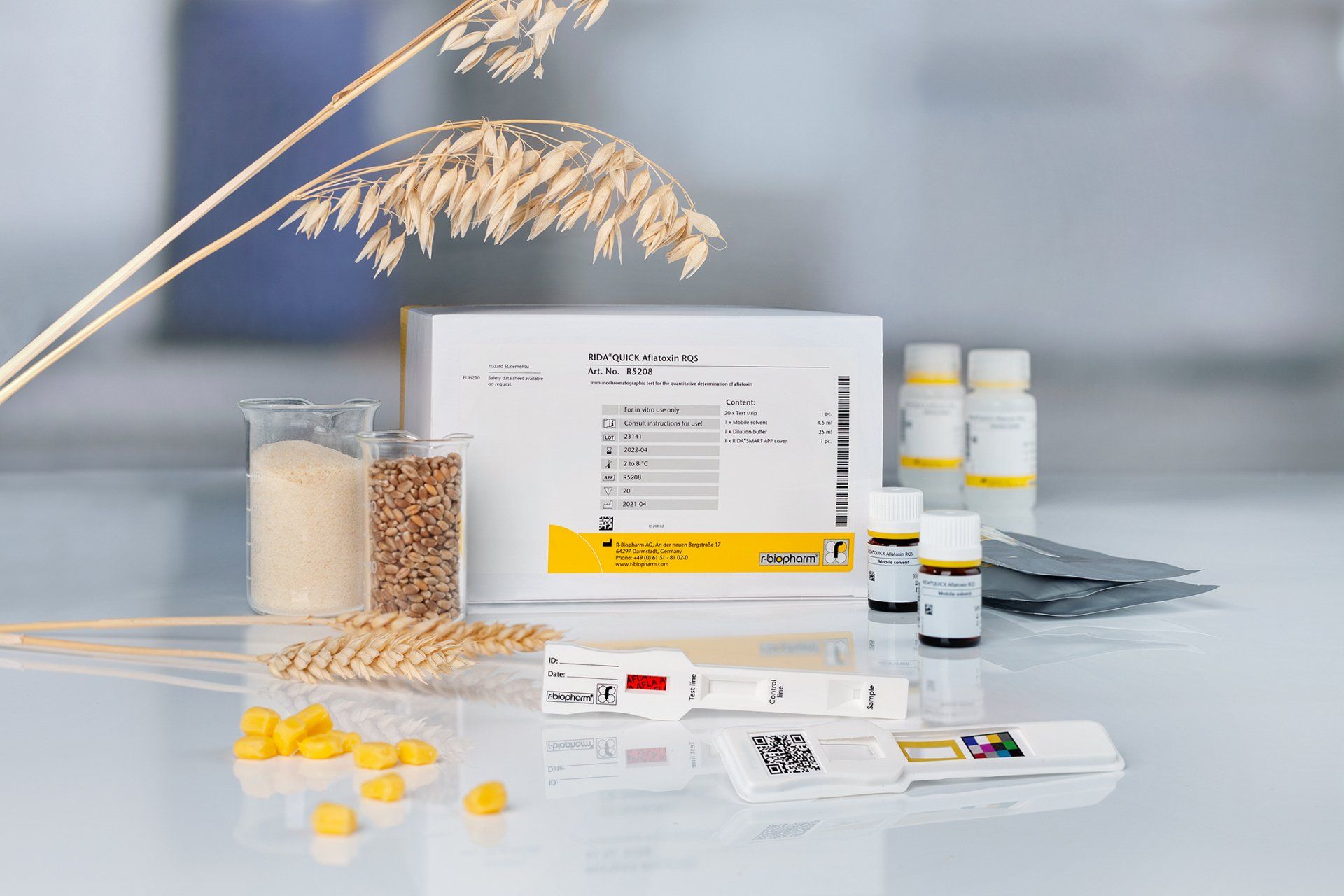
Lateral Flow Device – RIDA®QUICK
Lateral flow strips are easy to use and require little equipment. A sample is placed onto the sample pad. The sample diluent in the sample flow upwards through capillary action past the conjugate pad. Here the antigen i.e., the mycotoxin picks up a colloidal conjugate and carries it towards the test line where antibody bound onto nitrocellulose strip will pick up the antigen bound conjugate. The conjugate is coloured and show up as a coloured line. Conjugate that does not pick up any antigen will be carried past the test line to the control line where it will show up as another coloured line. Positive samples will have two coloured lines and negative one line at the control position.
RIDA®QUICK is an immunochromatographic tests for semi-quantitative (visual evaluation) or quantitative (evaluation with RIDA®SMART APP) analysis of mycotoxins. The format uses a lateral flow strip. It is available for alfa toxin, Don, zeralenone, fumonisin, T2/HT2. Before using the test strip, an extraction step with Ethanol/methanol is needed.
Benefits:
- Rapid result
- Simple sample preparation and assay procedure
- Allow to on-site decision about mycotoxin contamination
- Option for semi-quantitative/ fully-quantitative determination
RIDA®SMART APP
The
RIDA®SMART APP allows for quantitative measurements of mycotoxins with the use of lateral flow strips. This app is installed into a smartphone and scanning the strips with the smart phone camera allows the color intensity to be correlated to concentration of the mycotoxin. The app provides reliable and accurate results, which can be forwarded via e-mail, exported to a cloud server or easily sent to any supported printer
ELISA - RIDASCREEN®
RIDASCREEN® ELISA test for mycotoxins is based on competitive immunoassay. The base of the microtiter plate is coated with antibodies anti to the mycotoxin selected. Sample containing the antigen are added together with an antigen conjugate. The antigen competes with the conjugate bound antigen bound conjugate for a place on coated plate. A colour substrate is then added to the well and its colour strength will depend on the amount of mycotoxin. The more mycotoxin means that the colour will less intensely giving a curve with a negative gradient. The wells are read with a microtiter plate reader.
Uses the high specificity of antigen and antibody interaction to detect and quantify mycotoxins by photometric measurement. Final analysis is carried out with RIDA®SOFT Win.
Benefits:
- Quantitative result
- High sensitivity and specificity
- Fast and reliable
- Able to screen high sample numbers
- Walk away automation is available with Chemwell and Thunderbolt machine
Parameters:
Aflatoxin Total, Aflatoxin B1, Aflatoxin M1, DON, Zearalenon, Fumonisin, T-2/HT-2, T2, Citrinin

Sample Clean-up Column – Immunoaffinity Column
Uses the high specificity of antigen and antibody interaction to isolate, purify and concentrate mycotoxins in a wide range of commodities in conjunction with HPLC or LC-MS/MS.
Benefits:
- Multi analysis in conjunction with HPLC and LC-MS/MS
- Reduce background interference for improved accuracy of results
- Multiple applications
- Available in 1 ml and 3 ml format
Parameters:
Citrinin, Sterigmatocystin, Aflatoxin Total, Aflatoxin M, Zearalenone, Trichothecene P, T-2 & HT-2, Ochratoxin A, DON, Multitoxin DON, Zearalenone, T-2 and HT-2; Multitoxin for Aflatoxin and Ochratoxin; Multitoxin for Aflatoxin, Ochratoxin, Fumonisin; Multitoxin for Aflatoxin, Ochratoxin, Zearalenone
Certified Reference Material and Standard – Triology
Food and feed analysis imposes many challenges. Certified reference materials, quality control materials and participation in proficiency testing will strengthen your quality assurance. Trilogy Analytical Laboratory has the expertise to help you establish your quality control program and to keep up with industry regulations. Using our years of knowledge and experience, we can offer you a comprehensive line of quality products to meet the ever-changing needs of the mycotoxin industry.
Certified Reference Material:
Trilogy® Analytical Laboratory is an ISO 17034 accredited producer of mycotoxin reference materials and offers certified reference materials as well as quality control materials for mycotoxin analysis. Trilogy certified mycotoxin reference materials are available as naturally contaminated products, which have been certified according to ISO 17034. These certified reference materials may be used for validating methods and instrument calibration. Certified reference materials are available for various individual toxins as well as multiple mycotoxins, in various matrices and levels of contamination.
Certified Reference Standard and Analytical Standard:
Mycotoxin standards solutions are used routinely in mycotoxin analysis. They can be used to prepare a calibration curve for the HPLC system, ensuring accurate determination of the toxin. The standard solution can also be used to spike samples in order to check the extraction efficiency of the toxin from certain foods using a particular solvent, or alternatively, to spike sample extracts. R-Biopharm Indonesia offers ready to use standard solutions and in-situ generated mycotoxin standards from dry mycotoxin standards.












