Antibiotics Residues Testing Solutions

Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton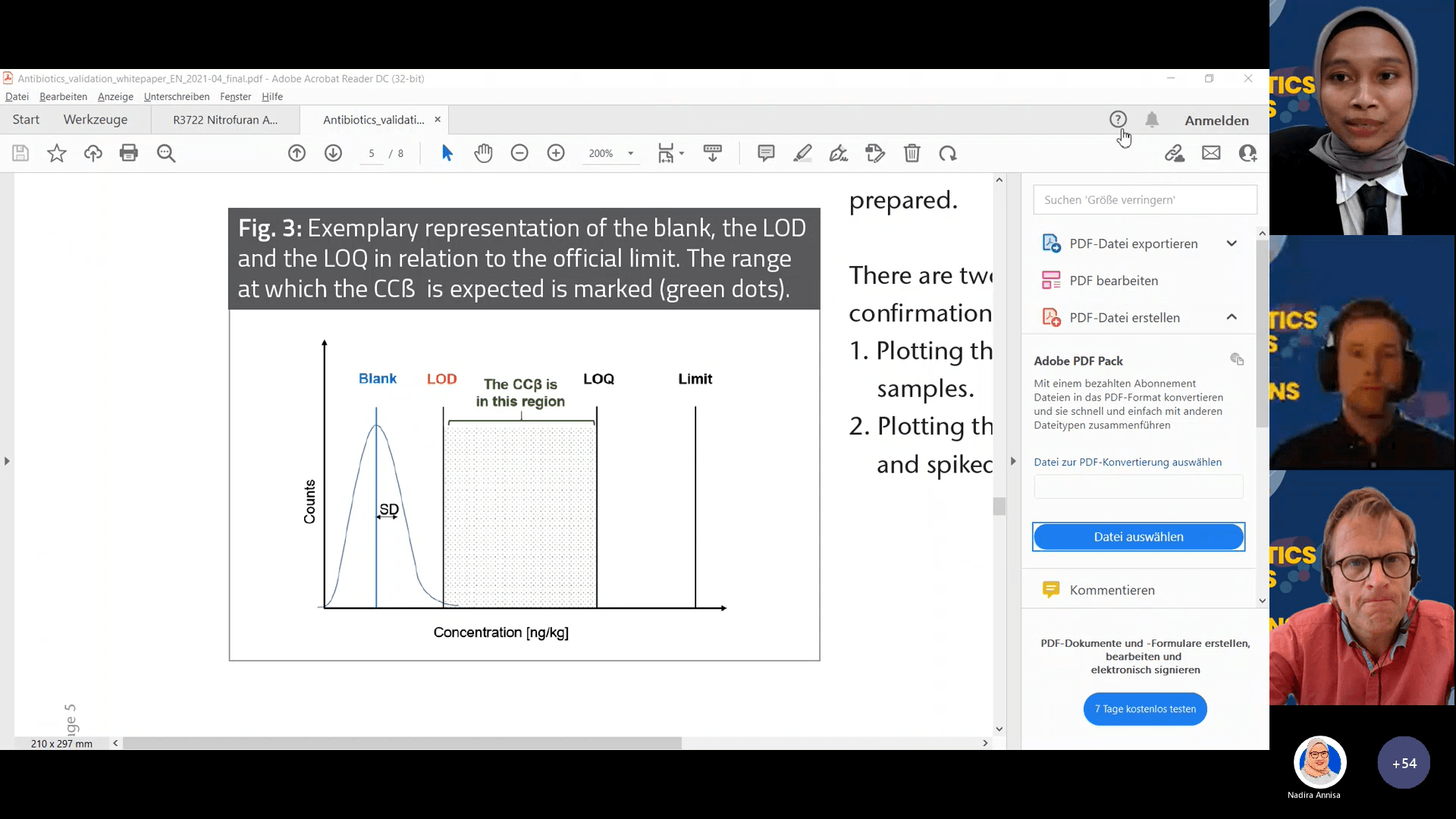
Slide title
Write your caption hereButton
Antibiotics are commonly used for treating bacterial infection. However, the antibiotics usage can be misused. This can lead to antibiotic resistance to the individual that consume the foods. Therefore, in order to give better solutions for antibiotics residue testing, Arasains collaborated with R-Biopharm, Germany conducting a webinar entitled “Antibiotics Residues Testing Solutions” on 29th June 2022.
The main topics of this webinar are about common regulation on major countries and solutions for antibiotics residues testing along with the correct sample preparations of antibiotics testing to get accurate results by Thomas Nick – Product Manager for Hormone and Antibiotics R-Biopharm, Marco Oteman – Product Manager for Hormones and Antibiotics Europroxima and Trilogys, and Eva Thonnes – Laboratory head technical support R-Biopharm. In this webinar, they also explained about ccβ that has been validated by R-Biopharm on antibiotics test kit. The difference between ccβ and LOQ is that the value LOQ is quantifiable, and the recovery is 75 % - 100%, meanwhile ccβ recovery is lower than that. The ccβ value is somewhere between LOD and LOQ.
BERITA TERBARU KAMI
Jelajahi Berita Terbaru Kami



To Get More Information
Don't Hesitate to Contact Us
Company Address
Rukan Kirana Boutique Office
Blok F3 no 12, Boulevard Raya No.1 Kelapa Gading. Jakarta Utara
Indonesia, 14240
Contact
+6221 4585 8833
info@arasains.co.id
Quick Link
Product Link
Copyright (c) 2021 PT Arasains. All Right Reserved





