Allergen Detection
Allergen
An allergen is a substance in food that causes a vigorous immune response in humans to a perceived threat that would otherwise be harmless. It stimulates a type 1 hypersensitive reaction through immunoglobulin E response. Usually this occurs only in individuals that have an absence of an enzyme to digest the particular food or they have celiac disease. It is estimated that worldwide about 8% of people have celiac disease.
The FDA (www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-questions-and-answers-regarding-food-allergens-edition-4) recognizes eight foods as commonly responsible for food allergies and they are peanuts, tree nuts, eggs, milk, shell fish, fish, wheat and their derivatives, soy and their derivatives, and sulfides. The EU regulation specifies 14 allergens as celery, cereals containing gluten including wheat, oats, rye, barley, crustaceans, eggs, fish, lupin, milk, mollusks, mustard, tree nuts, peanuts, sesame seeds, soy beans, Sulphur dioxide and sulfites.This is stated in the Regulation (EU) No 1169/2011 of the European Parliament and the Council of 25 October 2011 (eur-lex.europa.eu/legal-content/EN/ALL/).
The Federal food Drug and cosmetic act requires that the label of a food product disclose the presence of an ingredient derived from the eight allergens stated. If there is risk of a food being affected by cross contamination, the label should contain a warning of the allergen. Free from foods are special ranges of food made without allergens. Manufacturing of such products must be based on specific and rigorous controls to ensure that the final product is below the regulatory standards. Special efforts must be implemented to prevent cross contamination during manufacturing. As such cleaning of production equipment after a change of product becomes very important and a cleaning validation is needed. This is specifically emphasized in the Food Safety modernization act (www.fda.gov/food/food-safety-modernization-act-fsma/full-text-food-safety-modernization-act-fsma) good manufacturing practice and preventive controls. Sub section 117.3 of this act spell out allergen cross contact and sub part B establishes GMP guidelines to prevent allergen cross contamination. Sub section 117.135 establishes requirements for preventive controls. Sanitation controls, testing and cleaning validation is an important requisite for all the above regulations.
To carry a risk assessment of allergens it is prudent to carry out a thorough review of the allergen status of all ingredients and their processing conditions that contribute towards the allergen status of the final product. To this end, use of the Allergen bureau’s VITAL program (allergenbureau.net/wp-content/uploads/2019/10/Food-Industry-Guide-to-the-Voluntary-Incidental-Trace-Allergen-Labelling-VITAL-Program-Version-3.0_Web.pdf (Voluntary incidental trace allergen labelling) is useful. VITAL allows the assessment of likely sources of allergen cross contact from raw materials and the processing environment, plus an evaluation of the amount present and a review of the ability to reduce the allergenic material from all contributing sources. VITAL also provides for ongoing monitoring and verification of the risk assessment process to ensure any changes to the level of risk are acted upon without delay.
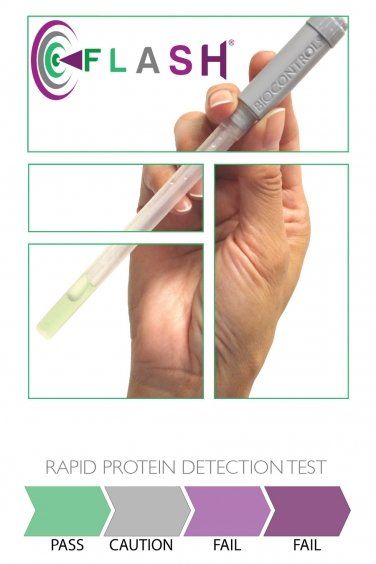
Protein Swab - FLASH®
FLASH® by Merck Biocontrol Indonesia is a room temperature-incubated total protein test that rapidly detects protein residues left on food contact surfaces after cleaning. Used as part of HACCP allergen control program, FLASH® supports process verification requirements that ensure cleaning methods, validated to effectively remove allergens, are consistently applied. FLASH® has been tested against common allergenic proteins including soy flour, gluten flour, milk powder, egg powder, peanut butter, roasted almonds, raw fish (cod), and raw shrimp.
Benefits:
- Easy to analysis with clear color interpretation
- Fast time to result, within 10 minutes
- Sensitivity detection down to 20 μg
- No instrumentation is required
- Can be used by all personnel without the need for extensive training
Lateral Flow Device - RIDA®QUICK and Bioavid
R-Biopharm Indonesia developed immunochromatographic tests for the semi-quantitative (visual evaluation) analysis of allergens, called RIDA®QUICK and bioavid.
Benefits:
- Rapid result
- Allow to on-site decision about allergen contamination
- Suitable for hygiene monitoring and end product testing
Parameters:
Gliadin (AOAC OMA), soya, milk, egg, crustacean, coconut, peanut, almond, hazelnut, walnut, Brazil nut, sesame, cashew, pistachio, mustard, macadamia nut
ELISA - RIDASCREEN®
RIDASCREEN® by R-Biopharm Indonesia use the high specificity of antigen and antibody interaction to detect and quantify allergens by photometric measurement.
Benefits:
- Quantitative result
- High sensitivity and specificity
- Fast and reliable
- Limits of detection or quantification at low mg/kg level
- Screening of many samples possible
Parameters:
Gliadin (AOAC OMA), soya, milk (AOAC), casein, β-lactoglobulin, egg, crustacean, peanut (AOAC), almond, hazelnut, macadamia, sesame, cashew, mustard.
PCR Technology - SureFood® Allergen
SureFood® from R-Biopharm Indonesia is a DNA based qualitative/quantitative analysis.
Benefits:
- Robust target molecule (DNA) in highly processed food samples
- Detection in many different food matrixes possible
- Highly specific system with minimum tendency to cross reactions
- Sensitivity in “low ppm” range, quantification possible
- One sample preparation (90 min) for 15 parameters
- Standardized handling and test procedure (1 – 2 hours)
- Qualitative/quantitative measurement
- Multiplexing is possible
Parameters:
Oat, mustard, lupin, celery, molluscs, fish, crustaceans, sesame, walnut, pistachio, pecan, macadamia, hazelnut, cashew, Brazil nut, almond, soya, gluten, multiplex soya/celery/mustard, customize kits available on request.
To Get More Information
Don't Hesitate to Contact Us
Company Address
Rukan Kirana Boutique Office
Blok F3 no 12, Boulevard Raya No.1 Kelapa Gading. Jakarta Utara
Indonesia, 14240
Contact
+6221 4585 8833
info@arasains.co.id
Quick Link
Product Link
Copyright (c) 2021 PT Arasains. All Right Reserved